How Fake Drugs Can Make It To Your Medicine Cabinet
This page is sponsored by The Turkewitz Law Firm
Counterfeit medications are increasing at an alarming rate in this country. They often target the people most at risk in our society – cancer, HIV or transplant patients – since they are the ones buying the most expensive drugs.
A single incident of counterfeit drugs may affect tens of thousands of people or more, such as the counterfeiting and recall of the popular cholesterol drug Lipitor (200,000 bottles) and the expensive anemia drug Procrit(an estimated 25,000 high risk people injected).
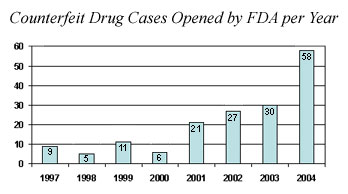
According to the Food and Drug Administration, there were about five investigations into fake drugs per year in the late 1990s. It shot up to 58 investigations in 2004 alone.
How does this happen? In February 2003, a Florida Grand Jury stated that, “an alarming percentage of the drugs flowing through the wholesale market have been illegally acquired. That is, they have been stolen from shipments, pharmacies, clinics, and hospitals; purchased on the black market from recipients and health care professionals who are defrauding insurance companies or Medicaid with bogus prescriptions; or illegally imported from overseas.”
Because the drugs can be purchased cheaper on this secondary market of unauthorized dealers than they can from the manufacturers, some wholesalers looking for greater profit margins buy them. Essentially, these wholesalers, unseen by the public, are playing Russian Roulette with the health, safety and well-being of the public by gambling (with the lives of sick patients) that the drugs they purchased on this secondary market are legitimate.
The Grand Jury wrote, “The fact that these criminals act with such callous disregard for human suffering is immoral and despicable, but we find that others involved in the industry bear responsibility by turning a blind eye to this activity for the sake of profit. By doing so, they enable these counterfeiters and re-labelers to thrive. Counterfeiters and re-labelers simply wouldn’t be in business if they did not have a steady supply of willing buyers in the marketplace.”
In October 2003, the Washington Post ran a full week series on the escalating problem of fake drugs, how they infiltrate our supply system, and how major wholesalers and pharmacies find them in their own stocks.
In July 2003, the FDA established an Anti-Counterfeiting Task Force to further address this issue. In February 2004 they issued their report. It was immediately denounced by Representative Steve Israel as being insufficient, as it called for voluntary efforts within the pharmaceutical industry, despite the prior 16 years of compliance failure.
Finally, in 2005, change started to sweep the industry.
In April, New York Attorney General Eliot Spitzer issued subpoenas to some of the largest wholesalers in the country as part of an investigation.

In May, Congressman Steve Israel announced “Tim Fagan’s Law” on the steps of New York’s City Hall, designed to toughen criminal penalties for counterfeiters and grant greater powers to the FDA to investigate and recall phony drugs. The bill is named for a 16 year old New Yorker who was injected with counterfeit drugs after an emergency liver transplant. Senator Charles Schumer introduced the Senate version in August — bipartisan legislation aimed at the escalating problem to better safeguard the public. Newsday and USA Today have both announced their support for Tim Fagan’s Law on their editorial pages.
Also in May, Dangerous Doses was released. This spellbinding exposé of the counterfeit drug market by former New York Times writer Katherine Eban, tracks Tim Fagan’s drugs through the hands of counterfeiters, into the mainstream of our pharmaceutical supply chain, and into the Fagan home. The “Five Horsemen of the Apocalypse”– a group of Florida investigators — are profiled as they hunt down the criminals who changed labels and resold the medications into the mainstream.
Eric Turkewitz, a New York personal injury attorney with numerous settlements and verdicts who represents Timothy Fagan, has spoken publicly before the FDA and pharmaceutical groups on the dangers of counterfeit drugs.
He has also written of the risks and dangers to governments that involve themselves with distributing drugs from unknown sources. (Counterfeit Drugs and the danger to Westchester, TheJournalNews.com, February 16, 2004)
Over the course of 2005, each of the “Big Three” in the wholesale distribution market, that collectively sell 90% of the nation’s wholesale pharmaceuticals, stated that they would no longer buy drugs out of the secondary drug market.
And in November, the House of Representatives’ subcommittee on Criminal Justice, Drug Policy, and Human Resources held a hearing on the rapidly increasing problem of counterfeit drugs.
Tim Fagan’s Law in currently pending in the House (H.R. 2345) and Senate (S. 1978) and can be tracked at these links.

On May 31, 2006, Mr. Turkewitz joined Assemblywoman Amy Paulin (D-Scarsdale), Senator Nick Spano (R-Yonkers), Katherine Eban (author of Dangerous Does) and Kevin Fagan (father of counterfeit drug victim Tim Fagan) in Scarsdale to help introduce tougher counterfeit drug legislation in New York. Those details can be read in the press release.
On June 9, 2006, after years of delay, the FDA announced that it would no longer stay the enforcement of regulations designed to track the “pedigree” of drugs as they move through the distribution system. While a large loophole still exists in that “authorized distributors of record” still don’t have to pass along pedigrees, the FDA regulations are, at long last, a step forward by the FDA. Tim Fagan’s Law, pending before both the House and Senate, will close down this final loophole and mandate pedigree papers from the manufacturer all the way down through the pharmacy, thereby making it exceedingly difficult for counterfeiters to breech our distribution system.
The continuing issues of counterfeit drugs and the pending legislation will be tracked as part of the New York Personal Injury Law Blog.
Actions in Florida (previously the site of much counterfeiting)
- Grand Jury Report from the State of Florida (February, 2003): Discussing how some in the pharmaceutical industry turn a “blind eye” to the counterfeiting problem, and engage in “willful blindness” in pursuit of profits. Web Link | Adobe PDF
- Florida Legislature — Justification Review: Counterfeit and Diverted Drugs Threaten Public Health and Waste State Dollars
Congressional Action
- Tim Fagan’s Law: The Counterfeit Drug Enforcement Act of 2005 sponsored by Congressman Steve Israel (D – NY).
- Increases criminal penalties. The current federal law is three years in prison. Israel’s bill increases penalties and includes up to life in prison.
- Mandates that a manufacturer must alert the FDA of a counterfeited drug in 2 days. Currently, there is no mandate. The pharmaceutical industry has said that it would voluntarily tell the FDA about counterfeited drugs within 5 business days.
- Provides the FDA with the authority to require companies to use anti-counterfeiting technology, as the technology becomes feasible and available.
- Mandates that the FDA implement the paper pedigree rule that was mandated in 1988 and has been postponed for 17 years. It also closes the “authorized wholesaler” loophole and includes manufacturers as needed to start the pedigree.
- Authorizes $60 million for spot-checking for counterfeits for each year between fiscal years 2006 and 2010.
- Authorizes $5 million for each year between fiscal years 2006 and 2010 for educating the public and health care professionals on how to identify counterfeit drugs.
- Provides recall authority to the FDA for prescription drugs. Currently, the FDA can only recall equipment and can only encourage private companies to recall their drugs.
- Authorizes the FDA to issue subpoenas with respect to preventing threats to public health.
- Press Release: Rep. Steve Israel (D-NY 02) Announces Groundbreaking Legislation, “Tim Fagan’s Law,” to Fight Counterfeit Prescription Drugs.
- Israel Makes Announcement Alongside Fagan Family, Author of New Book Exposing Dark Side of Pharmaceutical Trade, Investigators and Prosecutor
- A System Overwhelmed: The Avalanche of Imported, Counterfeit, and Unapproved Drugs into the U.S. The House Committee on Energy and Commerce, Subcommittee on Oversight and Investigations. (7/24/2003)
- Rep. Steve Israel Blasts FDA Anti-Counterfeit Drug Proposal As Insufficient
- Press Release: August 7, 2005, New York Senator Charles Schumer announced a Senate version of the counterfeit drug legislation pending in the House
Additional Counterfeit Drug Resource Links
- U.S. Food and Drug Administration Counterfeit Drug website
- Dangerous Doses: Kathereine Eban’s investigative look into the world of counterfeit prescription drugs, released May 9, 2005
- Rep. Steve Israel — The dangers of counterfeit drugs
- National Consumer’s League — Protect Yourself From Counterfeit Drugs
- Municipal Liability for distributing drugs, Eric Turkewitz, The Journal News, Feb. 16, 2004
